LSI researchers awarded funding through Spring 2024 CIHR Project Grant competition
July 24, 2024
Congratulations to all LSI researchers awarded funds in the CIHR Spring 2024 Project Grant competition! Please see below for a description of the funded projects (from CIHR site), including 4 priority announcement/bridge grants. In total, these grants were awarded > $2.4M from this competition.
Apart from the thousands of people who die suddenly of disordered heart rhythms every year, there are genetic diseases that cause serious heart rhythm disturbances, which affect the everyday lives of 1:2000 of the population, so about 20,000 Canadians. This research will help us understand the underlying properties of an ion-conducting protein in the heart that is responsible for some of the most common forms of inherited arrhythmias, and some cases of atrial fibrillation. By better understanding how the ion channel protein works normally and in disease, in the future we expect to design drugs to treat these chronic disease states, acute disorders of rhythm, and causes of sudden death. Many people need to wear implantable defibrillators, or take drugs to suppress their arrhythmias. This work is the first step towards developing more specific therapies to treat these heart conditions. We identified an important set of clinical cases where mutations in a specific region of one of the ion channels responsible for normal heart rhythm, called IKs, cause clinical arrhythmia syndromes. We will understand how and why these mutations cause disease by finding out at the molecular level what makes the channels less effective than they should be, or more effective than they should be. Both circumstances can have deleterious effects on stable heart rhythms. The genes for the channels will be expressed and particular structural features will be changed to mimic the effect of the arrhythmia disease. The modified channels will be studied electrically using micro-electrodes and advanced microscopy to determine exactly how different parts of the channels interact in the normal situation and in the presence of the arrhythmia-causing mutations. Advanced computer modeling will be used to understand results and gain insight into how the channel complexes normally work, and how this is modified in the disease-causing mutants.

Blood transfusion is a valuable tool in modern medicine and is very important to the health of surgical patients, accident victims, sickle cell, thalassemia and cancer patients. In the current blood banking protocols, blood typing ensures the safety of patients as mismatched blood transfusion can be very dangerous. In emergency conditions, the knowledge of blood group of patients may not be available. Also, in certain group of patients who receive frequent blood transfusions (25 to 50 units/year) (e.g., thalassemia, sickle cell and myelodysplastic syndromes patients) develop mild to severe immune reactions due to mismatch of minor antigens (>370 on red blood cells (RBCs) which are of proteins or carbohydrates in nature) and are not usually typed. Finding matched blood is challenging to these group of patients. In this project, we are developing a novel glycoengineering approach to address this important unmet clinical need by creating 'true' universal blood. We are developing novel and specific enzymes (proteins) identified from human gut bacteria from donors to cleave A and B antigens on RBC surface specifically to generate O type blood cells. Further we are developing a novel an enzymatic extension of sugar coat that is naturally present on RBCs to mask the immunogenicity of minor antigens which are responsible of immune reactions in many patients. We will also combine these two approaches to generate 'true universal donor blood' which can be transfused to anyone thereby improving the safety and supply for diverse patients. We use the state-of-the-art enzyme discovery, engineering and cell surface modification together with studies in human blood and animal studies to prove the concept. The development of such technology will be a fundamental advance and improve health of many Canadians. We also foresee that this technology can used other cell therapies and generation of universal organs for transplantation.

Leonard Foster
Co-investigator: Wilfred Jefferies (Michael Smith Laboratories)
Development and commercialization of a safe and effective Mpox subunit vaccine with global impact
Priority Announcement - Pandemic preparedness and health emergencies research
In a rapidly changing global landscape characterized by an expanding human population, diminishing habitats, and evolving disease patterns influenced by climate change, the likelihood of zoonotic diseases transitioning to humans has escalated. The recent COVID-19 pandemic underscored our unpreparedness, leading to significant health and economic impacts. While rapid solutions such as first-generation mRNA vaccines were developed, their payload capacity may not suffice to counter novel threats from Orthopoxviruses like monkeypox (Mpox). Concurrently, existing attenuated vaccines for poxviruses can be ineffective and, at times, induce adverse side effects, particularly in immunocompromised individuals. Here we propose to use innovative vaccine platforms to develop a safer and more effective Mpox vaccine than what is currently available by creating a subunit vaccine that retains a large number of immunologically important targets while removing the toxic side effects. In collaboration with Eyam Vaccines and Immunotherapeutics Ltd., we aim to create the first Mpox vaccine crafted by Canadian scientists and manufactured and distributed globally. This breakthrough will not only help protect vulnerable populations but also establish a rapid vaccine response system for other emerging pandemics caused by zoonotic diseases. Finally, the past pandemic has provided profound evidence that Canada should be in control of its own vaccine supplies and this proposal will facilitate this goal.

Pro-inflammatory platelet signaling: identifying novel therapeutic targets for periodontitis
Priority Announcement - IMHA: Oral Health
Periodontitis, or gum disease, is a very common infection of the mouth that causes chronically inflamed gum tissues and tooth loss. Periodontitis also increases the risk for serious diseases such as heart disease and diabetes. During periodontal infection, the immune cells that circulate in our bloodstream release signaling molecules known as cytokines. Some cytokines aggravate disease by driving up inflammation. The goal of this grant application is to study blood platelets, which are normally known as cells that control blood coagulation (clotting). However, platelets also release cytokines that contribute to inflammation. We will focus our studies on a specific cytokine called platelet factor 4 (PF4), and determine exactly how PF4 increases the "inflammatory" behaviour of other cells around the teeth and in the gum tissues. This information will give us a better idea of how platelets drive inflammation and may eventually lead to improved treatments for many chronic inflammatory diseases including arthritis, inflammatory bowel disease and periodontitis.

Elitza Tocheva
Co-investigator: Leonard Foster
Structural and functional characterization of the type VII secretion system in Mycobacteria
Priority Announcement - Antimicrobial resistance (Bridge Grant)
Mycobacteria, such as Mycobacterium tuberculosis (Mtb), are the deadliest bacterial pathogens, responsible for over 1.4 million deaths annually. One of the major reasons for the success of these pathogens is their unique and highly impermeable cell envelope, consisting of two membranes. While this envelope serves as a crucial barrier for survival during infection, conferring resistance to numerous antibiotics, it must simultaneously permit nutrient uptake and protein secretion for vital interactions with the host. To overcome these challenges, Mycobacteria have evolved a unique secretion system, the type VII secretion system (T7SS), as their main transport machinery that allows them to transport substrates, while maintaining the integrity of their cell envelope. Pathogenic mycobacteria, such as Mtb, have up to five subtypes of these secretion systems, named ESX-1 to ESX-5 and their substrates play distinct roles during infection, including preventing phagosome maturation, facilitating phagosome escape, inducing host cell death and manipulating the macrophage cytokine responses. While some substrates are important for virulence, others are essential for the uptake of specific metabolites that support mycobacterial growth. Therefore, the roles in virulence and viability make T7SS amongst the most versatile and important attributes of pathogenic mycobacteria. Elucidating the mechanism of T7S secretion and the role of individual effector proteins is therefore essential for understanding the success of pathogenic mycobacteria. Outcomes from this work could facilitate the identification of novel targets for drug and vaccine development.

Victor Viau
Priority Announcement - Sex and Gender in Health Research (Bridge Funding)
The stress hormone cortisol (aka hydrocortisone) is a glucocorticoid that is needed for development and function of many body systems, and is one of the most widely-prescribed medications. In our blood, cortisol is bound to a protein called corticosteroid-binding globulin (CBG) which carries it in the circulation and controls how it works on target tissues. When the blood levels of CBG are too low, there can be health problems, including poor ability to cope with stress, injury or inflammation, or chronic fatigue/pain and depression. Many of these traits are glucocorticoid dependent, but it remains unclear why these are manifested differently in males and females. A genetically-modified, CBG-deficient rat has been generated to test the role of CBG in modulating sex differences in immune, hormonal, and behavioural responses to stress.
Latest News

LSI PIs Sarah Hedtrich and Arun John Peter named Canada Research Chairs
March 28, 2025

Dr. Emilia Lim Named Dr. Victor Ling Terry Fox New Investigator
February 27, 2025
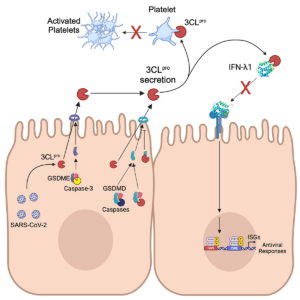
Unconventional secretion of the SARS-CoV-2 main protease opens the door for new extracellular biology in viral infection
February 11, 2025

LSI researchers awarded funding in Fall 2024 CIHR Project Grant competition
January 30, 2025

UBC evolutionary ecologist and LSI PI, Dr. Kayla King, awarded Arthur B. McDonald fellowship
November 21, 2024

UBC iGEM Team Wins Top Awards for Novel DNA-Based Data Storage Platform
November 13, 2024

Congratulations to LSI PIs receiving 2024 MSHRBC Scholar Awards!
October 1, 2024
LSI researchers awarded funding through Spring 2024 CIHR Project Grant competition
July 24, 2024

LSI researchers awarded over $9.3 million in CFI infrastructure funding
March 25, 2024

LSI Postdoctoral Researchers awarded the CIHR REDI Early Career Transition Award
March 12, 2024

Pioneering technology to simulate blood vessel networks in organ-on-chip models
February 29, 2024

5th Annual Canadian Metabolomics Conference: Integrating Metabolomics with Other Omics
February 28, 2024

LSI researchers awarded funding through Fall 2023 CIHR Project Grant competition
February 6, 2024

CBR/LSI Summer Studentship Program 2024
January 23, 2024

Dr. Kayla King named Canada Excellence Research Chair
November 22, 2023
