Rosin Lab
Research Focus Teams: Oral Health
Ongoing Projects
chevron_left
chevron_right
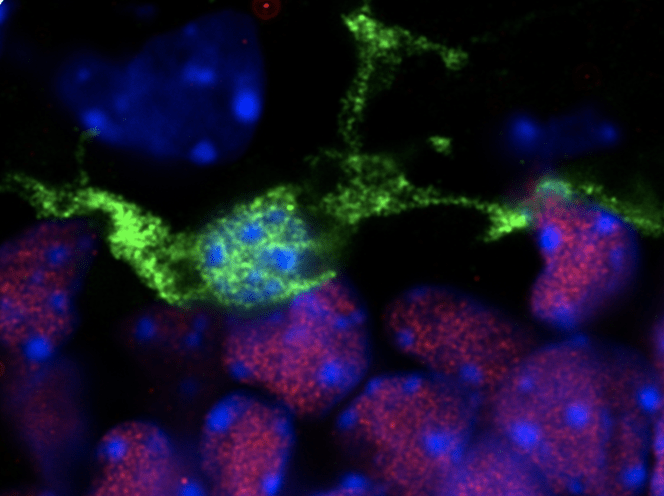
Investigating how maternal stress acts through microglia in a sex-specific manner to disrupt neurodevelopment
My Postdoctoral research and work from others provide evidence that microglia play important and unique roles in development of the hypothalamic brain region. Using single-cell RNA sequencing (scRNAseq), we previously identified four distinct populations of embryonic hypothalamic microglia, one of which is in direct contact with neural stem cells (NSCs) precisely at embryonic day 15.5 (E15.5)ΓÇöa time at which hypothalamic neurogenesis is terminating and gliogenic programs begin. As microglia are known to quickly respond to pathogenic insults, we exposed pregnant mice to intermittent periods of cold as a form of maternal stress to study how this gestational challenge impacts microglial physiology. Intriguingly, maternal stress elevated NSC-residing microglia numbers, pro-inflammatory cytokine and chemokine secretion, and significantly decreased the number of oxytocin neurons in the paraventricular nucleus (PVN), but only in male embryos. Furthermore, offspring exposed to maternal stress during gestation displayed social deficits, which showed a sex-specific dependency of microglia. This work demonstrates that embryonic microglia play an unappreciated role in translating maternal stressors to sex-specific perturbation of neurodevelopmental programs. Accordingly, Project 1 is targeted at providing mechanistic insight into the sex-specific responsiveness of microglia to maternal stress and further characterizing its impact on neurodevelopment.
Neuroscience Research:
Extensive proliferation of stem cells and early progenitors is required to generate all of the complex cell types and tissues that comprise the embryo. This often results in the overproduction of cells that need to be removed. Highly dynamic phagocytic immune cells, such as macrophages, exist across all embryonic tissues and are responsible for surveying their surrounding environment to remove dead or dying cells and dispose of cellular debris in order to maintain homeostasis. An emerging body of literature now demonstrates that microglia, the resident macrophages and phagocytic immune cells of the central nervous system (CNS), play an important role during neurodevelopment, beyond phagocytosis and cell removal.
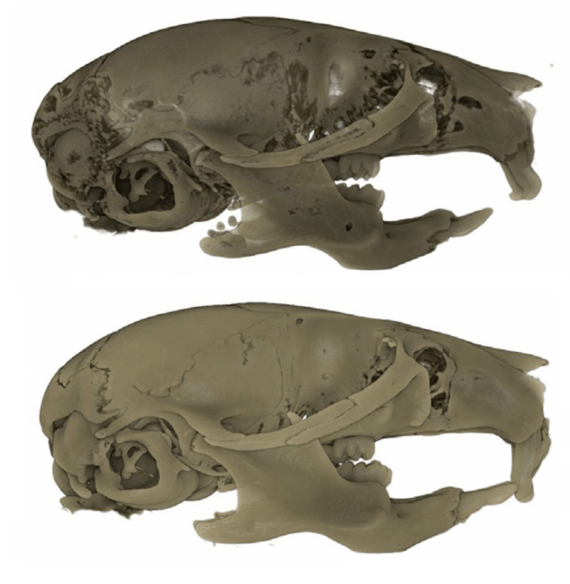
Characterizing the role CSF1R-expressing cells play in craniofacial development
My Postdoctoral research has shown that depleting CSF1R-expressing cells (i.e., microglia, macrophages, osteoclasts, etc.) during embryogenesis (E3.5 to birth) using the CSF1R inhibitor PLX5622 results in craniofacial and dental abnormalities in postnatal day 21 (P21) and P28 offspring. Specifically, depleting CSF1R-expressing cells during embryogenesis resulted in a distinct doming at the posterior aspect of the cranial vault and the absence of flattening of the mid-cranial vault in PLX5622 exposed animals. Moreover, the upper and lower incisors of PLX5622 exposed offspring lacked typical tooth curvature, displayed distinct notches in the enamel, and showed ectopic enamel ridges. Similarly, the first molars of PLX5622 exposed animals also displayed altered morphology, with a protuberance resembling an extra cusp. These phenotypes were observed in all male and female offspring exposed to the PLX5622 diet during gestation. Considering that macrophages and osteoclasts both express CSF1R, one or more of these cell types could be contributing to the disruptions observed in craniofacial morphogenesis. Accordingly, Project 2 is targeted at understanding how embryonic macrophages and osteoclasts contribute to normal craniofacial development during gestation.
Craniofacial Science Research:
Considering recent advances in the microglial field highlight the importance of these resident macrophages and phagocytic immune cells to brain development, a fundamental question is whether these same developmental roles evolved in other regions of the embryo, including craniofacial tissues.
Life Sciences Institute
Vancouver Campus
2350 Health Sciences Mall
Vancouver, BC Canada V6T 1Z3





